DNA
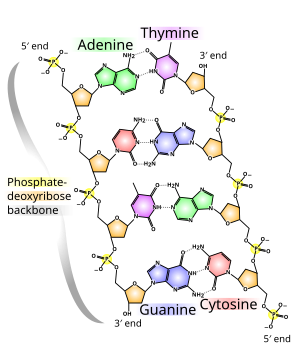
Deoxyribonucleic acid (DNA) is a molecule that encodes the genetic instructions used in the development and functioning of all known living organisms and many viruses. Along with RNA and proteins, DNA is one of the three majormacromolecules essential for all known forms of life. Most DNA molecules are double-stranded helices, consisting of two long biopolymers of simpler units called nucleotides—each nucleotide is composed of a nucleobase (guanine,adenine, thymine, and cytosine), recorded using the letters G, A, T, and C, as well as a backbone made of alternatingsugars (deoxyribose) and phosphate groups (related to phosphoric acid), with the nucleobases (G, A, T, C) attached to the sugars. DNA is well-suited for biological information storage, since the DNA backbone is resistant to cleavage and the double-stranded structure provides the molecule with a built-in duplicate of the encoded information.
The two strands of DNA run in opposite directions to each other and are therefore anti-parallel, one backbone being 3′ (three prime) and the other 5′ (five prime). This refers to the direction the 3rd and 5th carbon on the sugar molecule is facing. Attached to each sugar is one of four types of molecules called nucleobases (informally, bases). It is thesequence of these four nucleobases along the backbone that encodes genetic information. This information is read using the genetic code, which specifies the sequence of the amino acids within proteins. The code is read by copying stretches of DNA into the related nucleic acid RNA in a process called transcription.
Within cells, DNA is organized into long structures called chromosomes. During cell division these chromosomes are duplicated in the process of DNA replication, providing each cell its own complete set of chromosomes. Eukaryotic organisms (animals, plants, fungi, and protists) store most of their DNA inside the cell nucleus and some of their DNA inorganelles, such as mitochondria or chloroplasts.[1] In contrast, prokaryotes (bacteria and archaea) store their DNA only in the cytoplasm. Within the chromosomes, chromatin proteins such as histones compact and organize DNA. These compact structures guide the interactions between DNA and other proteins, helping control which parts of the DNA are transcribed.
The obsolete synonym "desoxyribonucleic acid" may occasionally be encountered, for example, in pre-1953 genetics.
Contents
[hide]Contents
[show]Properties
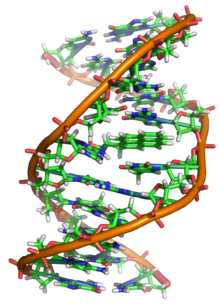
DNA is a long polymer made from repeating units called nucleotides.[2][3][4] DNA was first identified and isolated by Friedrich Miescher and the double helix structure of DNA was first discovered by James Watson and Francis Crick. The structure of DNA of all species comprises two helical chains each coiled round the same axis, and each with a pitch of 34 ångströms (3.4 nanometres) and a radius of 10 ångströms (1.0 nanometres).[5] According to another study, when measured in a particular solution, the DNA chain measured 22 to 26 ångströms wide (2.2 to 2.6 nanometres), and one nucleotide unit measured 3.3 Å (0.33 nm) long.[6] Although each individual repeating unit is very small, DNA polymers can be very large molecules containing millions of nucleotides. For instance, the largest human chromosome, chromosome number 1, consists of approximately 220 million base pairs[7] and is 85 mm long.
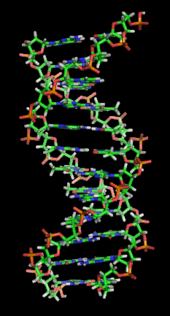
In living organisms DNA does not usually exist as a single molecule, but instead as a pair of molecules that are held tightly together.[8][9] These two long strands entwine like vines, in the shape of a double helix. The nucleotide repeats contain both the segment of the backbone of the molecule, which holds the chain together, and a nucleobase, which interacts with the other DNA strand in the helix. A nucleobase linked to a sugar is called a nucleoside and a base linked to a sugar and one or more phosphate groups is called a nucleotide. A polymer comprising multiple linked nucleotides (as in DNA) is called a polynucleotide.[10]
The backbone of the DNA strand is made from alternating phosphate and sugar residues.[11] The sugar in DNA is 2-deoxyribose, which is apentose (five-carbon) sugar. The sugars are joined together by phosphate groups that form phosphodiester bonds between the third and fifth carbon atoms of adjacent sugar rings. These asymmetric bonds mean a strand of DNA has a direction. In a double helix the direction of the nucleotides in one strand is opposite to their direction in the other strand: the strands areantiparallel. The asymmetric ends of DNA strands are called the 5′ (five prime) and 3′ (three prime) ends, with the 5′ end having a terminal phosphate group and the 3′ end a terminal hydroxyl group. One major difference between DNA and RNA is the sugar, with the 2-deoxyribose in DNA being replaced by the alternative pentose sugar ribose in RNA.[9]
The DNA double helix is stabilized primarily by two forces: hydrogen bonds between nucleotides and base-stacking interactions among aromatic nucleobases.[13] In the aqueous environment of the cell, the conjugated π bonds of nucleotide bases align perpendicular to the axis of the DNA molecule, minimizing their interaction with the solvation shell and therefore, the Gibbs free energy. The four bases found in DNA are adenine (abbreviated A), cytosine (C), guanine (G) and thymine (T). These four bases are attached to the sugar/phosphate to form the complete nucleotide, as shown for adenosine monophosphate.
Nucleobase classification
The nucleobases are classified into two types: the purines, A and G, being fused five- and six-membered heterocyclic compounds, and the pyrimidines, the six-membered rings C and T.[9] A fifth pyrimidine nucleobase, uracil (U), usually takes the place of thymine in RNA and differs from thymine by lacking a methyl group on its ring. In addition to RNA and DNA a large number of artificial nucleic acid analogues have also been created to study the properties of nucleic acids, or for use in biotechnology.[14]
Uracil is not usually found in DNA, occurring only as a breakdown product of cytosine. However in a number of bacteriophages – Bacillus subtilis bacteriophages PBS1 and PBS2 and Yersinia bacteriophage piR1-37 – thymine has been replaced by uracil.[15]
Base J (beta-d-glucopyranosyloxymethyluracil), a modified form of uracil, is also found in a number of organisms: the flagellates Diplonema andEuglena, and all the kinetoplastid genera[16] Biosynthesis of J occurs in two steps: in the first step a specific thymidine in DNA is converted into hydroxymethyldeoxyuridine; in the second HOMedU is glycosylated to form J.[17] Proteins that bind specifically to this base have been identified.[18][19][20] These proteins appear to be distant relatives of the Tet1 oncogene that is involved in the pathogenesis of acute myeloid leukemia.[21] J appears to act as a termination signal for RNA polymerase II.[22][23]
Grooves
Twin helical strands form the DNA backbone. Another double helix may be found tracing the spaces, or grooves, between the strands. These voids are adjacent to the base pairs and may provide a binding site. As the strands are not symmetrically located with respect to each other, the grooves are unequally sized. One groove, the major groove, is 22 Å wide and the other, the minor groove, is 12 Å wide.[24] The narrowness of the minor groove means that the edges of the bases are more accessible in the major groove. As a result, proteins like transcription factors that can bind to specific sequences in double-stranded DNA usually make contacts to the sides of the bases exposed in the major groove.[25] This situation varies in unusual conformations of DNA within the cell (see below), but the major and minor grooves are always named to reflect the differences in size that would be seen if the DNA is twisted back into the ordinary B form.
Base pairing
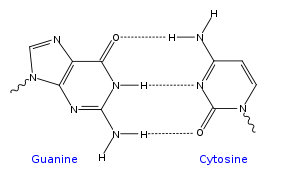
In a DNA double helix, each type of nucleobase on one strand bonds with just one type of nucleobase on the other strand. This is called complementary base pairing. Here, purines form hydrogen bonds to pyrimidines, with adenine bonding only to thymine in two hydrogen bonds, and cytosine bonding only to guanine in three hydrogen bonds. This arrangement of two nucleotides binding together across the double helix is called a base pair. As hydrogen bonds are not covalent, they can be broken and rejoined relatively easily. The two strands of DNA in a double helix can therefore be pulled apart like a zipper, either by a mechanical force or high temperature.[26] As a result of this complementarity, all the information in the double-stranded sequence of a DNA helix is duplicated on each strand, which is vital in DNA replication. Indeed, this reversible and specific interaction between complementary base pairs is critical for all the functions of DNA in living organisms.[3]
 |
 |
The two types of base pairs form different numbers of hydrogen bonds, AT forming two hydrogen bonds, and GC forming three hydrogen bonds (see figures, right). DNA with high GC-content is more stable than DNA with low GC-content.
As noted above, most DNA molecules are actually two polymer strands, bound together in a helical fashion by noncovalent bonds; this double stranded structure (dsDNA) is maintained largely by the intrastrand base stacking interactions, which are strongest for G,C stacks. The two strands can come apart – a process known as melting – to form two single-stranded DNA molecules (ssDNA) molecules. Melting occurs at high temperature, low salt and high pH (low pH also melts DNA, but since DNA is unstable due to acid depurination, low pH is rarely used).
The stability of the dsDNA form depends not only on the GC-content (% G,C basepairs) but also on sequence (since stacking is sequence specific) and also length (longer molecules are more stable). The stability can be measured in various ways; a common way is the "melting temperature", which is the temperature at which 50% of the ds molecules are converted to ss molecules; melting temperature is dependent on ionic strength and the concentration of DNA. As a result, it is both the percentage of GC base pairs and the overall length of a DNA double helix that determines the strength of the association between the two strands of DNA. Long DNA helices with a high GC-content have stronger-interacting strands, while short helices with high AT content have weaker-interacting strands.[27] In biology, parts of the DNA double helix that need to separate easily, such as the TATAAT Pribnow box in some promoters, tend to have a high AT content, making the strands easier to pull apart.[28]
In the laboratory, the strength of this interaction can be measured by finding the temperature necessary to break the hydrogen bonds, their melting temperature (also called Tm value). When all the base pairs in a DNA double helix melt, the strands separate and exist in solution as two entirely independent molecules. These single-stranded DNA molecules (ssDNA) have no single common shape, but some conformations are more stable than others.[29]
Sense and antisense
A DNA sequence is called "sense" if its sequence is the same as that of a messenger RNA copy that is translated into protein.[30] The sequence on the opposite strand is called the "antisense" sequence. Both sense and antisense sequences can exist on different parts of the same strand of DNA (i.e. both strands contain both sense and antisense sequences). In both prokaryotes and eukaryotes, antisense RNA sequences are produced, but the functions of these RNAs are not entirely clear.[31] One proposal is that antisense RNAs are involved in regulating gene expression through RNA-RNA base pairing.[32]
A few DNA sequences in prokaryotes and eukaryotes, and more in plasmids and viruses, blur the distinction between sense and antisense strands by having overlapping genes.[33] In these cases, some DNA sequences do double duty, encoding one protein when read along one strand, and a second protein when read in the opposite direction along the other strand. In bacteria, this overlap may be involved in the regulation of gene transcription,[34] while in viruses, overlapping genes increase the amount of information that can be encoded within the small viral genome.[35]
Supercoiling
DNA can be twisted like a rope in a process called DNA supercoiling. With DNA in its "relaxed" state, a strand usually circles the axis of the double helix once every 10.4 base pairs, but if the DNA is twisted the strands become more tightly or more loosely wound.[36] If the DNA is twisted in the direction of the helix, this is positive supercoiling, and the bases are held more tightly together. If they are twisted in the opposite direction, this is negative supercoiling, and the bases come apart more easily. In nature, most DNA has slight negative supercoiling that is introduced by enzymes called topoisomerases.[37] These enzymes are also needed to relieve the twisting stresses introduced into DNA strands during processes such as transcription and DNA replication.[38]
Alternate DNA structures
DNA exists in many possible conformations that include A-DNA, B-DNA, and Z-DNA forms, although, only B-DNA and Z-DNA have been directly observed in functional organisms.[11] The conformation that DNA adopts depends on the hydration level, DNA sequence, the amount and direction of supercoiling, chemical modifications of the bases, the type and concentration of metal ions, as well as the presence ofpolyamines in solution.[39]
The first published reports of A-DNA X-ray diffraction patterns — and also B-DNA — used analyses based on Patterson transforms that provided only a limited amount of structural information for oriented fibers of DNA.[40][41] An alternate analysis was then proposed by Wilkinset al., in 1953, for the in vivo B-DNA X-ray diffraction/scattering patterns of highly hydrated DNA fibers in terms of squares of Bessel functions.[42] In the same journal, James Watson and Francis Crick presented their molecular modeling analysis of the DNA X-ray diffraction patterns to suggest that the structure was a double-helix.[5]
Although the "B-DNA form" is most common under the conditions found in cells,[43] it is not a well-defined conformation but a family of related DNA conformations[44] that occur at the high hydration levels present in living cells. Their corresponding X-ray diffraction and scattering patterns are characteristic of molecular paracrystals with a significant degree of disorder.[45][46]
Compared to B-DNA, the A-DNA form is a wider right-handed spiral, with a shallow, wide minor groove and a narrower, deeper major groove. The A form occurs under non-physiological conditions in partially dehydrated samples of DNA, while in the cell it may be produced in hybrid pairings of DNA and RNA strands, as well as in enzyme-DNA complexes.[47][48]Segments of DNA where the bases have been chemically modified by methylation may undergo a larger change in conformation and adopt the Z form. Here, the strands turn about the helical axis in a left-handed spiral, the opposite of the more common B form.[49] These unusual structures can be recognized by specific Z-DNA binding proteins and may be involved in the regulation of transcription.[50]
Alternate DNA chemistry
For a number of years exobiologists have proposed the existence of a shadow biosphere, a postulated microbial biosphere of Earth that uses radically different biochemical and molecular processes than currently known life. One of the proposals was the existence of lifeforms that use arsenic instead of phosphorus in DNA. A report in 2010 of the possibility in the bacterium GFAJ-1, was announced,[51][51][52] though the research was disputed,[52][53] and evidence suggests the bacterium actively prevents the incorporation of arsenic into the DNA backbone and other biomolecules.[54]
Quadruplex structures
At the ends of the linear chromosomes are specialized regions of DNA called telomeres. The main function of these regions is to allow the cell to replicate chromosome ends using the enzyme telomerase, as the enzymes that normally replicate DNA cannot copy the extreme 3′ ends of chromosomes.[55] These specialized chromosome caps also help protect the DNA ends, and stop the DNA repair systems in the cell from treating them as damage to be corrected.[56] In human cells, telomeres are usually lengths of single-stranded DNA containing several thousand repeats of a simple TTAGGG sequence.[57]
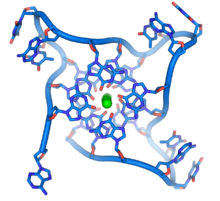
These guanine-rich sequences may stabilize chromosome ends by forming structures of stacked sets of four-base units, rather than the usual base pairs found in other DNA molecules. Here, four guanine bases form a flat plate and these flat four-base units then stack on top of each other, to form a stable G-quadruplex structure.[59] These structures are stabilized by hydrogen bonding between the edges of the bases and chelation of a metal ion in the centre of each four-base unit.[60] Other structures can also be formed, with the central set of four bases coming from either a single strand folded around the bases, or several different parallel strands, each contributing one base to the central structure.
In addition to these stacked structures, telomeres also form large loop structures called telomere loops, or T-loops. Here, the single-stranded DNA curls around in a long circle stabilized by telomere-binding proteins.[61] At the very end of the T-loop, the single-stranded telomere DNA is held onto a region of double-stranded DNA by the telomere strand disrupting the double-helical DNA and base pairing to one of the two strands. This triple-stranded structure is called a displacement loop or D-loop.[59]
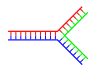 |
 |
| Single branch | Multiple branches |
Branched DNA
In DNA fraying occurs when non-complementary regions exist at the end of an otherwise complementary double-strand of DNA. However, branched DNA can occur if a third strand of DNA is introduced and contains adjoining regions able to hybridize with the frayed regions of the pre-existing double-strand. Although the simplest example of branched DNA involves only three strands of DNA, complexes involving additional strands and multiple branches are also possible.[62] Branched DNA can be used in nanotechnology to construct geometric shapes, see the section on uses in technology below.
Vibration
DNA may carry out low-frequency collective motion as observed by the Raman spectroscopy[63][64] and analyzed with a quasi-continuum model.[65][66]
Chemical modifications and altered DNA packaging
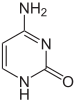 |
 |
 |
| cytosine | 5-methylcytosine | thymine |
Base modifications and DNA packaging
The expression of genes is influenced by how the DNA is packaged in chromosomes, in a structure called chromatin. Base modifications can be involved in packaging, with regions that have low or no gene expression usually containing high levels ofmethylation of cytosine bases. DNA packaging and its influence on gene expression can also occur by covalent modifications of the histone protein core around which DNA is wrapped in the chromatin structure or else by remodeling carried out by chromatin remodeling complexes (see Chromatin remodeling). There is, further, crosstalk between DNA methylation and histone modification, so they can coordinately affect chromatin and gene expression.[67]
For one example, cytosine methylation, produces 5-methylcytosine, which is important for X-chromosome inactivation.[68] The average level of methylation varies between organisms – the worm Caenorhabditis elegans lacks cytosine methylation, while vertebrates have higher levels, with up to 1% of their DNA containing 5-methylcytosine.[69] Despite the importance of 5-methylcytosine, it can deaminate to leave a thymine base, so methylated cytosines are particularly prone tomutations.[70] Other base modifications include adenine methylation in bacteria, the presence of 5-hydroxymethylcytosine in the brain,[71] and the glycosylation of uracil to produce the "J-base" in kinetoplastids.[72][73]
Damage
DNA can be damaged by many sorts of mutagens, which change the DNA sequence. Mutagens include oxidizing agents, alkylating agentsand also high-energy electromagnetic radiation such as ultraviolet light and X-rays. The type of DNA damage produced depends on the type of mutagen. For example, UV light can damage DNA by producing thymine dimers, which are cross-links between pyrimidine bases.[75] On the other hand, oxidants such as free radicals or hydrogen peroxide produce multiple forms of damage, including base modifications, particularly of guanosine, and double-strand breaks.[76] A typical human cell contains about 150,000 bases that have suffered oxidative damage.[77] Of these oxidative lesions, the most dangerous are double-strand breaks, as these are difficult to repair and can produce point mutations, insertions and deletions from the DNA sequence, as well as chromosomal translocations.[78] These mutations can causecancer. Because of inherent limitations in the DNA repair mechanisms, if humans lived long enough, they would all eventually develop cancer.[79][80] DNA damages that are naturally occurring, due to normal cellular processes that produce reactive oxygen species, the hydrolytic activities of cellular water, etc., also occur frequently. Although most of these damages are repaired, in any cell some DNA damage may remain despite the action of repair processes. These remaining DNA damages accumulate with age in mammalian postmitotic tissues. This accumulation appears to be an important underlying cause of aging.[81][82][83]
Many mutagens fit into the space between two adjacent base pairs, this is called intercalation. Most intercalators are aromatic and planar molecules; examples include ethidium bromide, acridines, daunomycin, and doxorubicin. For an intercalator to fit between base pairs, the bases must separate, distorting the DNA strands by unwinding of the double helix. This inhibits both transcription and DNA replication, causing toxicity and mutations.[84] As a result, DNA intercalators may be carcinogens, and in the case of thalidomide, a teratogen.[85]Others such as benzo[a]pyrene diol epoxide and aflatoxin form DNA adducts that induce errors in replication.[86] Nevertheless, due to their ability to inhibit DNA transcription and replication, other similar toxins are also used in chemotherapy to inhibit rapidly growing cancer cells.[87]
Biological functions
DNA usually occurs as linear chromosomes in eukaryotes, and circular chromosomes in prokaryotes. The set of chromosomes in a cell makes up its genome; the human genome has approximately 3 billion base pairs of DNA arranged into 46 chromosomes.[88] The information carried by DNA is held in the sequence of pieces of DNA called genes. Transmission of genetic information in genes is achieved via complementary base pairing. For example, in transcription, when a cell uses the information in a gene, the DNA sequence is copied into a complementary RNA sequence through the attraction between the DNA and the correct RNA nucleotides. Usually, this RNA copy is then used to make a matching protein sequence in a process called translation, which depends on the same interaction between RNA nucleotides. In alternative fashion, a cell may simply copy its genetic information in a process called DNA replication. The details of these functions are covered in other articles; here we focus on the interactions between DNA and other molecules that mediate the function of the genome.
Genes and genomes
Genomic DNA is tightly and orderly packed in the process called DNA condensation to fit the small available volumes of the cell. In eukaryotes, DNA is located in the cell nucleus, as well as small amounts in mitochondria and chloroplasts. In prokaryotes, the DNA is held within an irregularly shaped body in the cytoplasm called the nucleoid.[89] The genetic information in a genome is held within genes, and the complete set of this information in an organism is called its genotype. A gene is a unit of heredity and is a region of DNA that influences a particular characteristic in an organism. Genes contain an open reading frame that can be transcribed, as well as regulatory sequences such as promoters and enhancers, which control the transcription of the open reading frame.
In many species, only a small fraction of the total sequence of the genome encodes protein. For example, only about 1.5% of the human genome consists of protein-coding exons, with over 50% of human DNA consisting of non-coding repetitive sequences.[90] The reasons for the presence of so much noncoding DNA in eukaryotic genomes and the extraordinary differences in genome size, or C-value, among species represent a long-standing puzzle known as the "C-value enigma".[91] However, some DNA sequences that do not code protein may still encode functional non-coding RNA molecules, which are involved in the regulation of gene expression.[92]
Some noncoding DNA sequences play structural roles in chromosomes. Telomeres and centromeres typically contain few genes, but are important for the function and stability of chromosomes.[56][94] An abundant form of noncoding DNA in humans are pseudogenes, which are copies of genes that have been disabled by mutation.[95] These sequences are usually just molecular fossils, although they can occasionally serve as raw genetic material for the creation of new genes through the process of gene duplication and divergence.[96]
Transcription and translation
A gene is a sequence of DNA that contains genetic information and can influence the phenotype of an organism. Within a gene, the sequence of bases along a DNA strand defines a messenger RNA sequence, which then defines one or more protein sequences. The relationship between the nucleotide sequences of genes and the amino-acid sequences of proteins is determined by the rules of translation, known collectively as the genetic code. The genetic code consists of three-letter 'words' called codons formed from a sequence of three nucleotides (e.g. ACT, CAG, TTT).
In transcription, the codons of a gene are copied into messenger RNA by RNA polymerase. This RNA copy is then decoded by a ribosomethat reads the RNA sequence by base-pairing the messenger RNA to transfer RNA, which carries amino acids. Since there are 4 bases in 3-letter combinations, there are 64 possible codons (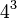 combinations). These encode the twenty standard amino acids, giving most amino acids more than one possible codon. There are also three 'stop' or 'nonsense' codons signifying the end of the coding region; these are the TAA, TGA and TAG codons.
combinations). These encode the twenty standard amino acids, giving most amino acids more than one possible codon. There are also three 'stop' or 'nonsense' codons signifying the end of the coding region; these are the TAA, TGA and TAG codons.
Replication
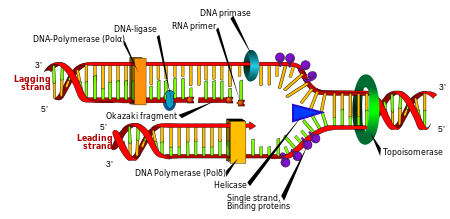
Cell division is essential for an organism to grow, but, when a cell divides, it must replicate the DNA in its genome so that the two daughter cells have the same genetic information as their parent. The double-stranded structure of DNA provides a simple mechanism for DNA replication. Here, the two strands are separated and then each strand's complementary DNA sequence is recreated by anenzyme called DNA polymerase. This enzyme makes the complementary strand by finding the correct base through complementary base pairing, and bonding it onto the original strand. As DNA polymerases can only extend a DNA strand in a 5′ to 3′ direction, different mechanisms are used to copy the antiparallel strands of the double helix.[97] In this way, the base on the old strand dictates which base appears on the new strand, and the cell ends up with a perfect copy of its DNA.
Interactions with proteins
All the functions of DNA depend on interactions with proteins. These protein interactions can be non-specific, or the protein can bind specifically to a single DNA sequence. Enzymes can also bind to DNA and of these, the polymerases that copy the DNA base sequence in transcription and DNA replication are particularly important.
DNA-binding proteins
 |
Structural proteins that bind DNA are well-understood examples of non-specific DNA-protein interactions. Within chromosomes, DNA is held in complexes with structural proteins. These proteins organize the DNA into a compact structure called chromatin. In eukaryotes this structure involves DNA binding to a complex of small basic proteins called histones, while in prokaryotes multiple types of proteins are involved.[98][99] The histones form a disk-shaped complex called a nucleosome, which contains two complete turns of double-stranded DNA wrapped around its surface. These non-specific interactions are formed through basic residues in the histones makingionic bonds to the acidic sugar-phosphate backbone of the DNA, and are therefore largely independent of the base sequence.[100]Chemical modifications of these basic amino acid residues include methylation, phosphorylation and acetylation.[101] These chemical changes alter the strength of the interaction between the DNA and the histones, making the DNA more or less accessible totranscription factors and changing the rate of transcription.[102] Other non-specific DNA-binding proteins in chromatin include the high-mobility group proteins, which bind to bent or distorted DNA.[103] These proteins are important in bending arrays of nucleosomes and arranging them into the larger structures that make up chromosomes.[104]
A distinct group of DNA-binding proteins are the DNA-binding proteins that specifically bind single-stranded DNA. In humans, replicationprotein A is the best-understood member of this family and is used in processes where the double helix is separated, including DNA replication, recombination and DNA repair.[105] These binding proteins seem to stabilize single-stranded DNA and protect it from forming stem-loops or being degraded by nucleases.
In contrast, other proteins have evolved to bind to particular DNA sequences. The most intensively studied of these are the various transcription factors, which are proteins that regulate transcription. Each transcription factor binds to one particular set of DNA sequences and activates or inhibits the transcription of genes that have these sequences close to their promoters. The transcription factors do this in two ways. Firstly, they can bind the RNA polymerase responsible for transcription, either directly or through other mediator proteins; this locates the polymerase at the promoter and allows it to begin transcription.[107] Alternatively, transcription factors can bind enzymes that modify the histones at the promoter. This changes the accessibility of the DNA template to the polymerase.[108]
As these DNA targets can occur throughout an organism's genome, changes in the activity of one type of transcription factor can affect thousands of genes.[109] Consequently, these proteins are often the targets of the signal transduction processes that control responses to environmental changes or cellular differentiation and development. The specificity of these transcription factors' interactions with DNA come from the proteins making multiple contacts to the edges of the DNA bases, allowing them to "read" the DNA sequence. Most of these base-interactions are made in the major groove, where the bases are most accessible.[25]
DNA-modifying enzymes
Nucleases and ligases
Nucleases are enzymes that cut DNA strands by catalyzing the hydrolysis of the phosphodiester bonds. Nucleases that hydrolyse nucleotides from the ends of DNA strands are called exonucleases, whileendonucleases cut within strands. The most frequently used nucleases in molecular biology are therestriction endonucleases, which cut DNA at specific sequences. For instance, the EcoRV enzyme shown to the left recognizes the 6-base sequence 5′-GATATC-3′ and makes a cut at the vertical line. In nature, these enzymes protect bacteria against phage infection by digesting the phage DNA when it enters the bacterial cell, acting as part of the restriction modification system.[111] In technology, these sequence-specific nucleases are used in molecular cloning and DNA fingerprinting.
Enzymes called DNA ligases can rejoin cut or broken DNA strands.[112] Ligases are particularly important in lagging strand DNA replication, as they join together the short segments of DNA produced at the replication fork into a complete copy of the DNA template. They are also used in DNA repair and genetic recombination.[112]
Topoisomerases and helicases
Topoisomerases are enzymes with both nuclease and ligase activity. These proteins change the amount of supercoiling in DNA. Some of these enzymes work by cutting the DNA helix and allowing one section to rotate, thereby reducing its level of supercoiling; the enzyme then seals the DNA break.[37] Other types of these enzymes are capable of cutting one DNA helix and then passing a second strand of DNA through this break, before rejoining the helix.[113] Topoisomerases are required for many processes involving DNA, such as DNA replication and transcription.[38]
Helicases are proteins that are a type of molecular motor. They use the chemical energy in nucleoside triphosphates, predominantly ATP, to break hydrogen bonds between bases and unwind the DNA double helix into single strands.[114] These enzymes are essential for most processes where enzymes need to access the DNA bases.
Polymerases
Polymerases are enzymes that synthesize polynucleotide chains from nucleoside triphosphates. The sequence of their products are copies of existing polynucleotide chains—which are called templates. These enzymes function by adding nucleotides onto the 3′ hydroxyl group of the previous nucleotide in a DNA strand. As a consequence, all polymerases work in a 5′ to 3′ direction.[115] In the active site of these enzymes, the incoming nucleoside triphosphate base-pairs to the template: this allows polymerases to accurately synthesize the complementary strand of their template. Polymerases are classified according to the type of template that they use.
In DNA replication, a DNA-dependent DNA polymerase makes a copy of a DNA sequence. Accuracy is vital in this process, so many of these polymerases have a proofreading activity. Here, the polymerase recognizes the occasional mistakes in the synthesis reaction by the lack of base pairing between the mismatched nucleotides. If a mismatch is detected, a 3′ to 5′ exonuclease activity is activated and the incorrect base removed.[116] In most organisms, DNA polymerases function in a large complex called the replisome that contains multiple accessory subunits, such as the DNA clamp or helicases.[117]
RNA-dependent DNA polymerases are a specialized class of polymerases that copy the sequence of an RNA strand into DNA. They include reverse transcriptase, which is a viralenzyme involved in the infection of cells by retroviruses, and telomerase, which is required for the replication of telomeres.[55][118] Telomerase is an unusual polymerase because it contains its own RNA template as part of its structure.[56]
Transcription is carried out by a DNA-dependent RNA polymerase that copies the sequence of a DNA strand into RNA. To begin transcribing a gene, the RNA polymerase binds to a sequence of DNA called a promoter and separates the DNA strands. It then copies the gene sequence into a messenger RNA transcript until it reaches a region of DNA called theterminator, where it halts and detaches from the DNA. As with human DNA-dependent DNA polymerases, RNA polymerase II, the enzyme that transcribes most of the genes in the human genome, operates as part of a large protein complex with multiple regulatory and accessory subunits.[119]
Genetic recombination
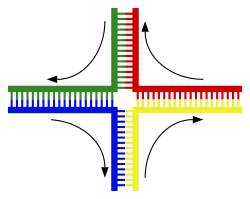 |
 |
A DNA helix usually does not interact with other segments of DNA, and in human cells the different chromosomes even occupy separate areas in the nucleus called "chromosome territories".[121] This physical separation of different chromosomes is important for the ability of DNA to function as a stable repository for information, as one of the few times chromosomes interact is during chromosomal crossover when they recombine. Chromosomal crossover is when two DNA helices break, swap a section and then rejoin.
Recombination allows chromosomes to exchange genetic information and produces new combinations of genes, which increases the efficiency of natural selection and can be important in the rapid evolution of new proteins.[122] Genetic recombination can also be involved in DNA repair, particularly in the cell's response to double-strand breaks.[123]
The most common form of chromosomal crossover is homologous recombination, where the two chromosomes involved share very similar sequences. Non-homologous recombination can be damaging to cells, as it can produce chromosomal translocationsand genetic abnormalities. The recombination reaction is catalyzed by enzymes known as recombinases, such as RAD51.[124] The first step in recombination is a double-stranded break caused by either an endonuclease or damage to the DNA.[125] A series of steps catalyzed in part by the recombinase then leads to joining of the two helices by at least one Holliday junction, in which a segment of a single strand in each helix is annealed to the complementary strand in the other helix. The Holliday junction is a tetrahedral junction structure that can be moved along the pair of chromosomes, swapping one strand for another. The recombination reaction is then halted by cleavage of the junction and re-ligation of the released DNA.[126]
Evolution
DNA contains the genetic information that allows all modern living things to function, grow and reproduce. However, it is unclear how long in the 4-billion-year history of life DNA has performed this function, as it has been proposed that the earliest forms of life may have used RNA as their genetic material.[127][128] RNA may have acted as the central part of early cell metabolism as it can both transmit genetic information and carry out catalysis as part of ribozymes.[129] This ancient RNA world where nucleic acid would have been used for both catalysis and genetics may have influenced the evolution of the current genetic code based on four nucleotide bases. This would occur, since the number of different bases in such an organism is a trade-off between a small number of bases increasing replication accuracy and a large number of bases increasing the catalytic efficiency of ribozymes.[130]
However, there is no direct evidence of ancient genetic systems, as recovery of DNA from most fossils is impossible. This is because DNA survives in the environment for less than one million years, and slowly degrades into short fragments in solution.[131] Claims for older DNA have been made, most notably a report of the isolation of a viable bacterium from a salt crystal 250 million years old,[132] but these claims are controversial.[133][134]
On 8 August 2011, a report, based on NASA studies with meteorites found on Earth, was published suggesting building blocks of DNA (adenine, guanine and related organic molecules) may have been formed extraterrestrially in outer space.[135][136][137]
Uses in technology
Genetic engineering
Methods have been developed to purify DNA from organisms, such as phenol-chloroform extraction, and to manipulate it in the laboratory, such as restriction digests and thepolymerase chain reaction. Modern biology and biochemistry make intensive use of these techniques in recombinant DNA technology. Recombinant DNA is a man-made DNA sequence that has been assembled from other DNA sequences. They can be transformed into organisms in the form of plasmids or in the appropriate format, by using a viral vector.[138] The genetically modified organisms produced can be used to produce products such as recombinant proteins, used in medical research,[139] or be grown inagriculture.[140][141]
Forensics
Forensic scientists can use DNA in blood, semen, skin, saliva or hair found at a crime scene to identify a matching DNA of an individual, such as a perpetrator. This process is formally termed DNA profiling, but may also be called "genetic fingerprinting". In DNA profiling, the lengths of variable sections of repetitive DNA, such as short tandem repeats and minisatellites, are compared between people. This method is usually an extremely reliable technique for identifying a matching DNA.[142] However, identification can be complicated if the scene is contaminated with DNA from several people.[143] DNA profiling was developed in 1984 by British geneticist Sir Alec Jeffreys,[144] and first used in forensic science to convict Colin Pitchfork in the 1988 Enderby murders case.[145]
The development of forensic science, and the ability to now obtain genetic matching on minute samples of blood, skin, saliva or hair has led to a re-examination of a number of cases. Evidence can now be uncovered that was not scientifically possible at the time of the original examination. Combined with the removal of the double jeopardy law in some places, this can allow cases to be reopened where previous trials have failed to produce sufficient evidence to convince a jury. People charged with serious crimes may be required to provide a sample of DNA for matching purposes. The most obvious defence to DNA matches obtained forensically is to claim that cross-contamination of evidence has taken place. This has resulted in meticulous strict handling procedures with new cases of serious crime. DNA profiling is also used to identify victims of mass casualty incidents.[146] As well as positively identifying bodies or body parts in serious accidents, DNA profiling is being successfully used to identify individual victims in mass war graves – matching to family members.
Bioinformatics
Bioinformatics involves the manipulation, searching, and data mining of biological data, and this includes DNA sequence data. The development of techniques to store and search DNA sequences have led to widely applied advances in computer science, especially string searching algorithms, machine learning and database theory.[147] String searching or matching algorithms, which find an occurrence of a sequence of letters inside a larger sequence of letters, were developed to search for specific sequences of nucleotides.[148] The DNA sequence may be aligned with other DNA sequences to identify homologous sequences and locate the specific mutations that make them distinct. These techniques, especiallymultiple sequence alignment, are used in studying phylogenetic relationships and protein function.[149] Data sets representing entire genomes' worth of DNA sequences, such as those produced by the Human Genome Project, are difficult to use without the annotations that identify the locations of genes and regulatory elements on each chromosome. Regions of DNA sequence that have the characteristic patterns associated with protein- or RNA-coding genes can be identified by gene finding algorithms, which allow researchers to predict the presence of particular gene products and their possible functions in an organism even before they have been isolated experimentally.[150] Entire genomes may also be compared, which can shed light on the evolutionary history of particular organism and permit the examination of complex evolutionary events.
DNA nanotechnology
DNA nanotechnology uses the unique molecular recognition properties of DNA and other nucleic acids to create self-assembling branched DNA complexes with useful properties.[151] DNA is thus used as a structural material rather than as a carrier of biological information. This has led to the creation of two-dimensional periodic lattices (both tile-based as well as using the "DNA origami" method) as well as three-dimensional structures in the shapes of polyhedra.[152] Nanomechanical devices and algorithmic self-assembly have also been demonstrated,[153] and these DNA structures have been used to template the arrangement of other molecules such as gold nanoparticles and streptavidin proteins.[154]
History and anthropology
Because DNA collects mutations over time, which are then inherited, it contains historical information, and, by comparing DNA sequences, geneticists can infer the evolutionary history of organisms, their phylogeny.[155]This field of phylogenetics is a powerful tool in evolutionary biology. If DNA sequences within a species are compared, population geneticists can learn the history of particular populations. This can be used in studies ranging from ecological genetics to anthropology; For example, DNA evidence is being used to try to identify the Ten Lost Tribes of Israel.[156][157]
DNA has also been used to look at modern family relationships, such as establishing family relationships between the descendants of Sally Hemings and Thomas Jefferson. This usage is closely related to the use of DNA in criminal investigations detailed above. Indeed, some criminal investigations have been solved when DNA from crime scenes has matched relatives of the guilty individual.[158]
Information storage
In a paper published in Nature in January, 2013, scientists from the European Bioinformatics Institute and Agilent Technologies proposed a mechanism to use DNA's ability to code information as a means of digital data storage. The group was able to encode 739 kilobytes of data into DNA code, synthesize the actual DNA, then sequence the DNA and decode the information back to its original form, with a reported 100% accuracy. The encoded information consisted of text files and audio files. A prior experiment was published in August 2012. It was conducted by researchers at Harvard University, where the text of a 54,000-word book was encoded in DNA.[159][160]
History of DNA research
DNA was first isolated by the Swiss physician Friedrich Miescher who, in 1869, discovered a microscopic substance in the pus of discarded surgical bandages. As it resided in the nuclei of cells, he called it "nuclein".[161] In 1878, Albrecht Kossel isolated the non-protein component of "nuclein", nucleic acid, and later isolated its five primary nucleobases.[162] In 1919, Phoebus Levene identified the base, sugar and phosphate nucleotide unit.[163] Levene suggested that DNA consisted of a string of nucleotide units linked together through the phosphate groups. However, Levene thought the chain was short and the bases repeated in a fixed order. In 1937 William Astbury produced the first X-ray diffraction patterns that showed that DNA had a regular structure.[164]
In 1927, Nikolai Koltsov proposed that inherited traits would be inherited via a "giant hereditary molecule" made up of "two mirror strands that would replicate in a semi-conservative fashion using each strand as a template".[165] In 1928, Frederick Griffith discovered that traits of the "smooth" form of Pneumococcus could be transferred to the "rough" form of the same bacteria by mixing killed "smooth" bacteria with the live "rough" form.[166] This system provided the first clear suggestion that DNA carries genetic information—the Avery–MacLeod–McCarty experiment—when Oswald Avery, along with coworkers Colin MacLeod and Maclyn McCarty, identified DNA as the transforming principle in 1943.[167] DNA's role in heredity was confirmed in 1952, when Alfred Hershey and Martha Chase in the Hershey–Chase experiment showed that DNA is the genetic material of the T2 phage.[168]
In 1953, James Watson and Francis Crick suggested what is now accepted as the first correct double-helix model of DNA structure in the journal Nature.[5] Their double-helix, molecular model of DNA was then based on a single X-ray diffraction image (labeled as "Photo 51")[169] taken by Rosalind Franklin and Raymond Gosling in May 1952, as well as the information that the DNA bases are paired — also obtained through private communications from Erwin Chargaff in the previous years. Chargaff's rules played a very important role in establishing double-helix configurations for B-DNA as well as A-DNA.
Experimental evidence supporting the Watson and Crick model was published in a series of five articles in the same issue of Nature.[170] Of these, Franklin and Gosling's paper was the first publication of their own X-ray diffraction data and original analysis method that partially supported the Watson and Crick model;[41][171] this issue also contained an article on DNA structure by Maurice Wilkins and two of his colleagues, whose analysis and in vivo B-DNA X-ray patterns also supported the presence in vivo of the double-helical DNA configurations as proposed by Crick and Watson for their double-helix molecular model of DNA in the previous two pages of Nature.[42] In 1962, after Franklin's death, Watson, Crick, and Wilkins jointly received the Nobel Prize in Physiology or Medicine.[172] Nobel Prizes were awarded only to living recipients at the time. A debate continues about who should receive credit for the discovery.[173]
In an influential presentation in 1957, Crick laid out the central dogma of molecular biology, which foretold the relationship between DNA, RNA, and proteins, and articulated the "adaptor hypothesis".[174] Final confirmation of the replication mechanism that was implied by the double-helical structure followed in 1958 through the Meselson–Stahl experiment.[175] Further work by Crick and coworkers showed that the genetic code was based on non-overlapping triplets of bases, called codons, allowing Har Gobind Khorana, Robert W. Holley and Marshall Warren Nirenberg to decipher the genetic code.[176] These findings represent the birth of molecular biology.
See also
- Autosome
- Crystallography
- DNA-encoded chemical library
- DNA microarray
- DNA sequencing
- Genetic disorder
- Haplotype
- List of nucleic acid simulation software- Nucleic acid modeling
- Meiosis
- Nucleic acid double helix
- Nucleic acid notation
- Phosphoramidite
- Southern blot
- X-ray scattering techniques
- Proteopedia DNA
References
- ^ Russell, Peter (2001). iGenetics. New York: Benjamin Cummings. ISBN 0-8053-4553-1.
- ^ Saenger, Wolfram (1984). Principles of Nucleic Acid Structure. New York: Springer-Verlag. ISBN 0-387-90762-9.
- ^ a b Alberts, Bruce; Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts and Peter Walters (2002).Molecular Biology of the Cell; Fourth Edition. New York and London: Garland Science. ISBN 0-8153-3218-1.OCLC 145080076 48122761 57023651 69932405.
- ^ Butler, John M. (2001). Forensic DNA Typing. Elsevier.ISBN 978-0-12-147951-0. OCLC 223032110 45406517.pp. 14–15.
- ^ a b c Watson J.D. and Crick F.H.C. (1953). "A Structure for Deoxyribose Nucleic Acid" (PDF). Nature 171(4356): 737–738. Bibcode:1953Natur.171..737W.doi:10.1038/171737a0. PMID 13054692.
- ^ Mandelkern M, Elias J, Eden D, Crothers D (1981). "The dimensions of DNA in solution". J Mol Biol 152 (1): 153–61. doi:10.1016/0022-2836(81)90099-1.PMID 7338906.
- ^ Gregory S; Barlow, KF; McLay, KE; Kaul, R; Swarbreck, D; Dunham, A; Scott, CE; Howe, KL; Woodfine, K (2006). "The DNA sequence and biological annotation of human chromosome 1". Nature 441 (7091): 315–21.Bibcode:2006Natur.441..315G.doi:10.1038/nature04727. PMID 16710414.
- ^ Watson J.D. and Crick F.H.C. (1953). "A Structure for Deoxyribose Nucleic Acid" (PDF). Nature 171 (4356): 737–738. Bibcode:1953Natur.171..737W.doi:10.1038/171737a0. PMID 13054692. Retrieved 4 May 2009.
- ^ a b c Berg J., Tymoczko J. and Stryer L. (2002)Biochemistry. W. H. Freeman and Company ISBN 0-7167-4955-6
- ^ Abbreviations and Symbols for Nucleic Acids, Polynucleotides and their Constituents IUPAC-IUB Commission on Biochemical Nomenclature (CBN). Retrieved 3 January 2006.
- ^ a b Ghosh A, Bansal M (2003). "A glossary of DNA structures from A to Z". Acta Crystallogr D 59 (4): 620–6.doi:10.1107/S0907444903003251. PMID 12657780.
- ^ Created from PDB 1D65
- ^ Yakovchuk P, Protozanova E, Frank-Kamenetskii MD (2006). "Base-stacking and base-pairing contributions into thermal stability of the DNA double helix". Nucleic Acids Res. 34 (2): 564–74. doi:10.1093/nar/gkj454.PMC 1360284. PMID 16449200.
- ^ Verma S, Eckstein F (1998). "Modified oligonucleotides: synthesis and strategy for users". Annu. Rev. Biochem.67: 99–134. doi:10.1146/annurev.biochem.67.1.99.PMID 9759484.
- ^ Kiljunen S, Hakala K, Pinta E, Huttunen S, Pluta P, Gador A, Lönnberg H, Skurnik M (2005). "Yersiniophage phiR1-37 is a tailed bacteriophage having a 270 kb DNA genome with thymidine replaced by deoxyuridine".Microbiology 151 (12): 4093–4102.doi:10.1099/mic.0.28265-0. PMID 16339954.
- ^ Simpson L (1998). "A base called J". Proc Natl Acad Sci USA 95 (5): 2037–2038.doi:10.1073/pnas.95.5.2037. PMC 33841.PMID 9482833.
- ^ Borst P, Sabatini R (2008). "Base J: discovery, biosynthesis, and possible functions". Annual review of microbiology 62: 235–51.doi:10.1146/annurev.micro.62.081307.162750.PMID 18729733.
- ^ Cross M, Kieft R, Sabatini R, Wilm M, de Kort M, der Marel GA, van Boom JH, van Leeuwen F, Borst P et al.(1999). "The modified base J is the target for a novel DNA-binding protein in kinetoplastid protozoans". EMBO J 18(22): 6573–6581. doi:10.1093/emboj/18.22.6573.PMC 1171720. PMID 10562569.
- ^ DiPaolo C, Kieft R, Cross M, Sabatini R (2005). "Regulation of trypanosome DNA glycosylation by a SWI2/SNF2-like protein". Mol Cell 17 (3): 441–451.doi:10.1016/j.molcel.2004.12.022. PMID 15694344.
- ^ Vainio S, Genest PA, ter Riet B, van Luenen H, Borst P (2009). "Evidence that J-binding protein 2 is a thymidine hydroxylase catalyzing the first step in the biosynthesis of DNA base J". Molecular and biochemical parasitology 164(2): 157–61. doi:10.1016/j.molbiopara.2008.12.001.PMID 19114062.
- ^ Iyer LM, Tahiliani M, Rao A, Aravind L (2009). "Prediction of novel families of enzymes involved in oxidative and other complex modifications of bases in nucleic acids".Cell Cycle 8 (11): 1698–1710.doi:10.4161/cc.8.11.8580. PMC 2995806.PMID 19411852.
- ^ Van Luenen HG, Farris C, Jan S, Genest PA, Tripathi P, Velds A, Kerkhoven RM, Nieuwland M, Haydock A et al.(2012). "Leishmania". Cell 150 (5): 909–921.doi:10.1016/j.cell.2012.07.030. PMC 3684241.PMID 22939620.
- ^ Hazelbaker DZ, Buratowski S (2012). "Transcription: base J blocks the way". Curr Biol 22 (22): R960–2.doi:10.1016/j.cub.2012.10.010. PMC 3648658.PMID 23174300.
- ^ Wing R, Drew H, Takano T, Broka C, Tanaka S, Itakura K, Dickerson R (1980). "Crystal structure analysis of a complete turn of B-DNA". Nature 287 (5784): 755–8.Bibcode:1980Natur.287..755W.doi:10.1038/287755a0. PMID 7432492.
- ^ a b Pabo C, Sauer R (1984). "Protein-DNA recognition".Annu Rev Biochem 53: 293–321.doi:10.1146/annurev.bi.53.070184.001453.PMID 6236744.
- ^ Clausen-Schaumann H, Rief M, Tolksdorf C, Gaub H (2000). "Mechanical stability of single DNA molecules".Biophys J 78 (4): 1997–2007.Bibcode:2000BpJ....78.1997C. doi:10.1016/S0006-3495(00)76747-6. PMC 1300792. PMID 10733978.
- ^ Chalikian T, Völker J, Plum G, Breslauer K (1999). "A more unified picture for the thermodynamics of nucleic acid duplex melting: A characterization by calorimetric and volumetric techniques". Proc Natl Acad Sci USA 96 (14): 7853–8. Bibcode:1999PNAS...96.7853C.doi:10.1073/pnas.96.14.7853. PMC 22151.PMID 10393911.
- ^ deHaseth P, Helmann J (1995). "Open complex formation by Escherichia coli RNA polymerase: the mechanism of polymerase-induced strand separation of double helical DNA". Mol Microbiol 16 (5): 817–24.doi:10.1111/j.1365-2958.1995.tb02309.x.PMID 7476180.
- ^ Isaksson J, Acharya S, Barman J, Cheruku P, Chattopadhyaya J (2004). "Single-stranded adenine-rich DNA and RNA retain structural characteristics of their respective double-stranded conformations and show directional differences in stacking pattern". Biochemistry 43(51): 15996–6010. doi:10.1021/bi048221v.PMID 15609994.
- ^ Designation of the two strands of DNA JCBN/NC-IUB Newsletter 1989. Retrieved 7 May 2008
- ^ Hüttenhofer A, Schattner P, Polacek N (2005). "Non-coding RNAs: hope or hype?". Trends Genet 21 (5): 289–97. doi:10.1016/j.tig.2005.03.007. PMID 15851066.
- ^ Munroe S (2004). "Diversity of antisense regulation in eukaryotes: multiple mechanisms, emerging patterns". J Cell Biochem 93 (4): 664–71. doi:10.1002/jcb.20252.PMID 15389973.
- ^ Makalowska I, Lin C, Makalowski W (2005). "Overlapping genes in vertebrate genomes". Comput Biol Chem 29 (1): 1–12. doi:10.1016/j.compbiolchem.2004.12.006.PMID 15680581.
- ^ Johnson Z, Chisholm S (2004). "Properties of overlapping genes are conserved across microbial genomes". Genome Res 14 (11): 2268–72.doi:10.1101/gr.2433104. PMC 525685.PMID 15520290.
- ^ Lamb R, Horvath C (1991). "Diversity of coding strategies in influenza viruses". Trends Genet 7 (8): 261–6.doi:10.1016/0168-9525(91)90326-L. PMID 1771674.
- ^ Benham C, Mielke S (2005). "DNA mechanics". Annu Rev Biomed Eng 7: 21–53.doi:10.1146/annurev.bioeng.6.062403.132016.PMID 16004565.
- ^ a b Champoux J (2001). "DNA topoisomerases: structure, function, and mechanism". Annu Rev Biochem70: 369–413. doi:10.1146/annurev.biochem.70.1.369.PMID 11395412.
- ^ a b Wang J (2002). "Cellular roles of DNA topoisomerases: a molecular perspective". Nat Rev Mol Cell Biol 3 (6): 430–40. doi:10.1038/nrm831.PMID 12042765.
- ^ Basu H, Feuerstein B, Zarling D, Shafer R, Marton L (1988). "Recognition of Z-RNA and Z-DNA determinants by polyamines in solution: experimental and theoretical studies". J Biomol Struct Dyn 6 (2): 299–309.doi:10.1080/07391102.1988.10507714.PMID 2482766.
- ^ Franklin RE, Gosling RG (6 March 1953). "The Structure of Sodium Thymonucleate Fibres I. The Influence of Water Content". Acta Crystallogr 6 (8–9): 673–7.doi:10.1107/S0365110X53001939. Archived from the original on 2007-06-12.
Franklin RE, Gosling RG (1953). "The structure of sodium thymonucleate fibres. II. The cylindrically symmetrical Patterson function". Acta Crystallogr 6 (8–9): 678–85.doi:10.1107/S0365110X53001940. - ^ a b Franklin, Rosalind and Gosling, Raymond (1953)."Molecular Configuration in Sodium Thymonucleate. Franklin R. and Gosling R.G" (PDF). Nature 171(4356): 740–1. Bibcode:1953Natur.171..740F.doi:10.1038/171740a0. PMID 13054694.
- ^ a b Wilkins M.H.F., A.R. Stokes A.R. & Wilson, H.R. (1953). "Molecular Structure of Deoxypentose Nucleic Acids" (PDF). Nature 171 (4356): 738–740.Bibcode:1953Natur.171..738W.doi:10.1038/171738a0. PMID 13054693.
- ^ Leslie AG, Arnott S, Chandrasekaran R, Ratliff RL (1980). "Polymorphism of DNA double helices". J. Mol. Biol. 143 (1): 49–72. doi:10.1016/0022-2836(80)90124-2. PMID 7441761.
- ^ Baianu, I.C. (1980). "Structural Order and Partial Disorder in Biological systems". Bull. Math. Biol. 42 (4): 137–141.http://cogprints.org/3822/
- ^ Hosemann R., Bagchi R.N., Direct analysis of diffraction by matter, North-Holland Publs., Amsterdam – New York, 1962.
- ^ Baianu, I.C. (1978). "X-ray scattering by partially disordered membrane systems". Acta Crystallogr A 34 (5): 751–753. Bibcode:1978AcCrA..34..751B.doi:10.1107/S0567739478001540.
- ^ Wahl M, Sundaralingam M (1997). "Crystal structures of A-DNA duplexes". Biopolymers 44 (1): 45–63.doi:10.1002/(SICI)1097-0282(1997)44:1<45::AID-BIP4>3.0.CO;2-#. PMID 9097733.
- ^ Lu XJ, Shakked Z, Olson WK (2000). "A-form conformational motifs in ligand-bound DNA structures". J. Mol. Biol. 300 (4): 819–40. doi:10.1006/jmbi.2000.3690.PMID 10891271.
- ^ Rothenburg S, Koch-Nolte F, Haag F (2001). "DNA methylation and Z-DNA formation as mediators of quantitative differences in the expression of alleles".Immunol Rev 184: 286–98. doi:10.1034/j.1600-065x.2001.1840125.x. PMID 12086319.
- ^ Oh D, Kim Y, Rich A (2002). "Z-DNA-binding proteins can act as potent effectors of gene expression in vivo".Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16666–71.Bibcode:2002PNAS...9916666O.doi:10.1073/pnas.262672699. PMC 139201.PMID 12486233.
- ^ a b "Arsenic-loving bacteria may help in hunt for alien life". BBC News. 2 December 2010. Retrieved 2 December 2010.
- ^ a b Bortman, Henry (2 December 2010). "Arsenic-Eating Bacteria Opens New Possibilities for Alien Life".Space.Com web site (Space.com). Retrieved 2 December 2010.
- ^ Katsnelson, Alla (2 December 2010). "Arsenic-eating microbe may redefine chemistry of life". Nature News.doi:10.1038/news.2010.645.
- ^ <Please add first missing authors to populate metadata.>, Cressey (3 October 2012). "'Arsenic-life' Bacterium Prefers Phosphorus after all". Nature News.doi:10.1038/news.2012.11520.
- ^ a b Greider C, Blackburn E (1985). "Identification of a specific telomere terminal transferase activity in Tetrahymena extracts". Cell 43 (2 Pt 1): 405–13.doi:10.1016/0092-8674(85)90170-9. PMID 3907856.
- ^ a b c Nugent C, Lundblad V (1998). "The telomerase reverse transcriptase: components and regulation". Genes Dev 12 (8): 1073–85. doi:10.1101/gad.12.8.1073.PMID 9553037.
- ^ Wright W, Tesmer V, Huffman K, Levene S, Shay J (1997). "Normal human chromosomes have long G-rich telomeric overhangs at one end". Genes Dev 11 (21): 2801–9. doi:10.1101/gad.11.21.2801. PMC 316649.PMID 9353250.
- ^ Created from NDB UD0017
- ^ a b Burge S, Parkinson G, Hazel P, Todd A, Neidle S (2006). "Quadruplex DNA: sequence, topology and structure". Nucleic Acids Res 34 (19): 5402–15.doi:10.1093/nar/gkl655. PMC 1636468.PMID 17012276.
- ^ Parkinson G, Lee M, Neidle S (2002). "Crystal structure of parallel quadruplexes from human telomeric DNA". Nature417 (6891): 876–80. doi:10.1038/nature755.PMID 12050675.
- ^ Griffith J, Comeau L, Rosenfield S, Stansel R, Bianchi A, Moss H, de Lange T (1999). "Mammalian telomeres end in a large duplex loop". Cell 97 (4): 503–14.doi:10.1016/S0092-8674(00)80760-6.PMID 10338214.
- ^ Seeman NC (2005). "DNA enables nanoscale control of the structure of matter". Q. Rev. Biophys. 38 (4): 363–71. doi:10.1017/S0033583505004087. PMC 3478329.PMID 16515737.
- ^ Painter PC, Mosher LE, Rhoads C (1982). "Low-frequency modes in the Raman spectra of proteins".Biopolymers 21 (7): 1469–72.doi:10.1002/bip.360210715. PMID 7115900.
- ^ Urabe H, Tominaga Y, Kubota K (1983). "Experimental evidence of collective vibrations in DNA double helix (Raman spectroscopy)". Journal of Chemical Physics 78(10): 5937–5939. Bibcode:1983JChPh..78.5937U.doi:10.1063/1.444600.
- ^ Chou KC (1984). "Low-frequency vibrations of DNA molecules". Biochem. J. 221 (1): 27–31.PMC 1143999. PMID 6466317.
- ^ Chou KC, Maggiora GM, Mao B (1989). "Quasi-continuum models of twist-like and accordion-like low-frequency motions in DNA". Biophys. J. 56 (2): 295–305.Bibcode:1989BpJ....56..295C. doi:10.1016/S0006-3495(89)82676-1. PMC 1280479. PMID 2775828.
- ^ Hu Q, Rosenfeld MG. (2012) Epigenetic regulation of human embryonic stem cells. Front Genet. 3:238. doi: 10.3389/fgene.2012.00238. PMID 23133442
- ^ Klose R, Bird A (2006). "Genomic DNA methylation: the mark and its mediators". Trends Biochem Sci 31 (2): 89–97. doi:10.1016/j.tibs.2005.12.008. PMID 16403636.
- ^ Bird A (2002). "DNA methylation patterns and epigenetic memory". Genes Dev 16 (1): 6–21.doi:10.1101/gad.947102. PMID 11782440.
- ^ Walsh C, Xu G (2006). "Cytosine methylation and DNA repair". Curr Top Microbiol Immunol. Current Topics in Microbiology and Immunology 301: 283–315.doi:10.1007/3-540-31390-7_11. ISBN 3-540-29114-8.PMID 16570853.
- ^ Kriaucionis S, Heintz N (2009). "The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain". Science 324 (5929): 929–30.Bibcode:2009Sci...324..929K.doi:10.1126/science.1169786. PMC 3263819.PMID 19372393.
- ^ Ratel D, Ravanat J, Berger F, Wion D (2006). "N6-methyladenine: the other methylated base of DNA".BioEssays 28 (3): 309–15. doi:10.1002/bies.20342.PMC 2754416. PMID 16479578.
- ^ Gommers-Ampt J, Van Leeuwen F, de Beer A, Vliegenthart J, Dizdaroglu M, Kowalak J, Crain P, Borst P (1993). "beta-D-glucosyl-hydroxymethyluracil: a novel modified base present in the DNA of the parasitic protozoan T. brucei". Cell 75 (6): 1129–36.doi:10.1016/0092-8674(93)90322-H. PMID 8261512.
- ^ Created from PDB 1JDG
- ^ Douki T, Reynaud-Angelin A, Cadet J, Sage E (2003). "Bipyrimidine photoproducts rather than oxidative lesions are the main type of DNA damage involved in the genotoxic effect of solar UVA radiation". Biochemistry 42(30): 9221–6. doi:10.1021/bi034593c.PMID 12885257.
- ^ Cadet J, Delatour T, Douki T, Gasparutto D, Pouget J, Ravanat J, Sauvaigo S (1999). "Hydroxyl radicals and DNA base damage". Mutat Res 424 (1–2): 9–21.doi:10.1016/S0027-5107(99)00004-4.PMID 10064846.
- ^ Beckman KB, Ames BN (1997). "Oxidative decay of DNA". J. Biol. Chem. 272 (32): 19633–6.doi:10.1074/jbc.272.32.19633. PMID 9289489.
- ^ Valerie K, Povirk L (2003). "Regulation and mechanisms of mammalian double-strand break repair". Oncogene 22(37): 5792–812. doi:10.1038/sj.onc.1206679.PMID 12947387.
- ^ Johnson, George (28 December 2010). "Unearthing Prehistoric Tumors, and Debate". The New York Times. "If we lived long enough, sooner or later we all would get cancer."
- ^ Alberts, B, Johnson A, Lewis J, et al. (2002). "The Preventable Causes of Cancer". Molecular biology of the cell (4th ed.). New York: Garland Science. ISBN 0-8153-4072-9. "A certain irreducible background incidence of cancer is to be expected regardless of circumstances: mutations can never be absolutely avoided, because they are an inescapable consequence of fundamental limitations on the accuracy of DNA replication, as discussed in Chapter 5. If a human could live long enough, it is inevitable that at least one of his or her cells would eventually accumulate a set of mutations sufficient for cancer to develop."
- ^ Bernstein H, Payne CM, Bernstein C, Garewal H, Dvorak K (2008). Cancer and aging as consequences of un-repaired DNA damage. In: New Research on DNA Damages (Editors: Honoka Kimura and Aoi Suzuki) Nova Science Publishers, Inc., New York, Chapter 1, pp. 1-47. open access, but read onlyhttps://www.novapublishers.com/catalog/product_info.php?products_id=43247 ISBN 1604565810 ISBN 978-1604565812
- ^ Hoeijmakers JH (October 2009). "DNA damage, aging, and cancer". N. Engl. J. Med. 361 (15): 1475–85.doi:10.1056/NEJMra0804615. PMID 19812404.
- ^ Freitas AA, de Magalhães JP (2011). "A review and appraisal of the DNA damage theory of ageing". Mutat. Res.728 (1–2): 12–22. doi:10.1016/j.mrrev.2011.05.001.PMID 21600302.
- ^ Ferguson L, Denny W (1991). "The genetic toxicology of acridines". Mutat Res 258 (2): 123–60. doi:10.1016/0165-1110(91)90006-H. PMID 1881402.
- ^ Stephens T, Bunde C, Fillmore B (2000). "Mechanism of action in thalidomide teratogenesis". Biochem Pharmacol59 (12): 1489–99. doi:10.1016/S0006-2952(99)00388-3. PMID 10799645.
- ^ Jeffrey A (1985). "DNA modification by chemical carcinogens". Pharmacol Ther 28 (2): 237–72.doi:10.1016/0163-7258(85)90013-0. PMID 3936066.
- ^ Braña M, Cacho M, Gradillas A, de Pascual-Teresa B, Ramos A (2001). "Intercalators as anticancer drugs". Curr Pharm Des 7 (17): 1745–80.doi:10.2174/1381612013397113. PMID 11562309.
- ^ Venter J; Adams, MD; Myers, EW; Li, PW; Mural, RJ; Sutton, GG; Smith, HO; Yandell, M; Evans, CA (2001). "The sequence of the human genome". Science 291(5507): 1304–51. Bibcode:2001Sci...291.1304V.doi:10.1126/science.1058040. PMID 11181995.
- ^ Thanbichler M, Wang S, Shapiro L (2005). "The bacterial nucleoid: a highly organized and dynamic structure". J Cell Biochem 96 (3): 506–21. doi:10.1002/jcb.20519.PMID 15988757.
- ^ Wolfsberg T, McEntyre J, Schuler G (2001). "Guide to the draft human genome". Nature 409 (6822): 824–6.doi:10.1038/35057000. PMID 11236998.
- ^ Gregory T (2005). "The C-value enigma in plants and animals: a review of parallels and an appeal for partnership". Ann Bot (Lond) 95 (1): 133–46.doi:10.1093/aob/mci009. PMID 15596463.
- ^ The ENCODE Project Consortium (2007). "Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project". Nature 447(7146): 799–816. Bibcode:2007Natur.447..799B.doi:10.1038/nature05874. PMC 2212820.PMID 17571346.
- ^ Created from PDB 1MSW
- ^ Pidoux A, Allshire R (2005). "The role of heterochromatin in centromere function". Philos Trans R Soc Lond B Biol Sci 360 (1455): 569–79. doi:10.1098/rstb.2004.1611.PMC 1569473. PMID 15905142.
- ^ Harrison P, Hegyi H, Balasubramanian S, Luscombe N, Bertone P, Echols N, Johnson T, Gerstein M (2002)."Molecular Fossils in the Human Genome: Identification and Analysis of the Pseudogenes in Chromosomes 21 and 22". Genome Res 12 (2): 272–80.doi:10.1101/gr.207102. PMC 155275.PMID 11827946.
- ^ Harrison P, Gerstein M (2002). "Studying genomes through the aeons: protein families, pseudogenes and proteome evolution". J Mol Biol 318 (5): 1155–74.doi:10.1016/S0022-2836(02)00109-2.PMID 12083509.
- ^ Albà M (2001). "Replicative DNA polymerases".Genome Biol 2 (1): reviews3002.1–reviews3002.4.doi:10.1186/gb-2001-2-1-reviews3002.PMC 150442. PMID 11178285.
- ^ Sandman K, Pereira S, Reeve J (1998). "Diversity of prokaryotic chromosomal proteins and the origin of the nucleosome". Cell Mol Life Sci 54 (12): 1350–64.doi:10.1007/s000180050259. PMID 9893710.
- ^ Dame RT (2005). "The role of nucleoid-associated proteins in the organization and compaction of bacterial chromatin". Mol. Microbiol. 56 (4): 858–70.doi:10.1111/j.1365-2958.2005.04598.x.PMID 15853876.
- ^ Luger K, Mäder A, Richmond R, Sargent D, Richmond T (1997). "Crystal structure of the nucleosome core particle at 2.8 A resolution". Nature 389 (6648): 251–60.Bibcode:1997Natur.389..251L. doi:10.1038/38444.PMID 9305837.
- ^ Jenuwein T, Allis C (2001). "Translating the histone code". Science 293 (5532): 1074–80.doi:10.1126/science.1063127. PMID 11498575.
- ^ Ito T (2003). "Nucleosome assembly and remodelling".Curr Top Microbiol Immunol. Current Topics in Microbiology and Immunology 274: 1–22. doi:10.1007/978-3-642-55747-7_1. ISBN 978-3-540-44208-0.PMID 12596902.
- ^ Thomas J (2001). "HMG1 and 2: architectural DNA-binding proteins". Biochem Soc Trans 29 (Pt 4): 395–401.doi:10.1042/BST0290395. PMID 11497996.
- ^ Grosschedl R, Giese K, Pagel J (1994). "HMG domain proteins: architectural elements in the assembly of nucleoprotein structures". Trends Genet 10 (3): 94–100.doi:10.1016/0168-9525(94)90232-1. PMID 8178371.
- ^ Iftode C, Daniely Y, Borowiec J (1999). "Replication protein A (RPA): the eukaryotic SSB". Crit Rev Biochem Mol Biol 34 (3): 141–80.doi:10.1080/10409239991209255. PMID 10473346.
- ^ Created from PDB 1LMB
- ^ Myers L, Kornberg R (2000). "Mediator of transcriptional regulation". Annu Rev Biochem 69: 729–49.doi:10.1146/annurev.biochem.69.1.729.PMID 10966474.
- ^ Spiegelman B, Heinrich R (2004). "Biological control through regulated transcriptional coactivators". Cell 119(2): 157–67. doi:10.1016/j.cell.2004.09.037.PMID 15479634.
- ^ Li Z, Van Calcar S, Qu C, Cavenee W, Zhang M, Ren B (2003). "A global transcriptional regulatory role for c-Myc in Burkitt's lymphoma cells". Proc Natl Acad Sci USA100 (14): 8164–9. Bibcode:2003PNAS..100.8164L.doi:10.1073/pnas.1332764100. PMC 166200.PMID 12808131.
- ^ Created from PDB 1RVA
- ^ Bickle T, Krüger D (1993). "Biology of DNA restriction".Microbiol Rev 57 (2): 434–50. PMC 372918.PMID 8336674.
- ^ a b Doherty A, Suh S (2000). "Structural and mechanistic conservation in DNA ligases". Nucleic Acids Res 28(21): 4051–8. doi:10.1093/nar/28.21.4051.PMC 113121. PMID 11058099.
- ^ Schoeffler A, Berger J (2005). "Recent advances in understanding structure-function relationships in the type II topoisomerase mechanism". Biochem Soc Trans 33 (Pt 6): 1465–70. doi:10.1042/BST20051465.PMID 16246147.
- ^ Tuteja N, Tuteja R (2004). "Unraveling DNA helicases. Motif, structure, mechanism and function". Eur J Biochem271 (10): 1849–63. doi:10.1111/j.1432-1033.2004.04094.x. PMID 15128295.
- ^ Joyce C, Steitz T (1995). "Polymerase structures and function: variations on a theme?". J Bacteriol 177 (22): 6321–9. PMC 177480. PMID 7592405.
- ^ Hubscher U, Maga G, Spadari S (2002). "Eukaryotic DNA polymerases". Annu Rev Biochem 71: 133–63.doi:10.1146/annurev.biochem.71.090501.150041.PMID 12045093.
- ^ Johnson A, O'Donnell M (2005). "Cellular DNA replicases: components and dynamics at the replication fork". Annu Rev Biochem 74: 283–315.doi:10.1146/annurev.biochem.73.011303.073859.PMID 15952889.
- ^ Tarrago-Litvak L, Andréola M, Nevinsky G, Sarih-Cottin L, Litvak S (1 May 1994). "The reverse transcriptase of HIV-1: from enzymology to therapeutic intervention".FASEB J 8 (8): 497–503. PMID 7514143.
- ^ Martinez E (2002). "Multi-protein complexes in eukaryotic gene transcription". Plant Mol Biol 50 (6): 925–47. doi:10.1023/A:1021258713850. PMID 12516863.
- ^ Created from PDB 1M6G
- ^ Cremer T, Cremer C (2001). "Chromosome territories, nuclear architecture and gene regulation in mammalian cells". Nat Rev Genet 2 (4): 292–301.doi:10.1038/35066075. PMID 11283701.
- ^ Pál C, Papp B, Lercher M (2006). "An integrated view of protein evolution". Nat Rev Genet 7 (5): 337–48.doi:10.1038/nrg1838. PMID 16619049.
- ^ O'Driscoll M, Jeggo P (2006). "The role of double-strand break repair – insights from human genetics". Nat Rev Genet 7 (1): 45–54. doi:10.1038/nrg1746.PMID 16369571.
- ^ Vispé S, Defais M (1997). "Mammalian Rad51 protein: a RecA homologue with pleiotropic functions". Biochimie 79(9–10): 587–92. doi:10.1016/S0300-9084(97)82007-X.PMID 9466696.
- ^ Neale MJ, Keeney S (2006). "Clarifying the mechanics of DNA strand exchange in meiotic recombination". Nature442 (7099): 153–8. Bibcode:2006Natur.442..153N.doi:10.1038/nature04885. PMID 16838012.
- ^ Dickman M, Ingleston S, Sedelnikova S, Rafferty J, Lloyd R, Grasby J, Hornby D (2002). "The RuvABC resolvasome". Eur J Biochem 269 (22): 5492–501.doi:10.1046/j.1432-1033.2002.03250.x.PMID 12423347.
- ^ Joyce G (2002). "The antiquity of RNA-based evolution".Nature 418 (6894): 214–21. doi:10.1038/418214a.PMID 12110897.
- ^ Orgel L (2004). "Prebiotic chemistry and the origin of the RNA world". Crit Rev Biochem Mol Biol 39 (2): 99–123.doi:10.1080/10409230490460765. PMID 15217990.
- ^ Davenport R (2001). "Ribozymes. Making copies in the RNA world". Science 292 (5520): 1278.doi:10.1126/science.292.5520.1278a.PMID 11360970.
- ^ Szathmáry E (1992). "What is the optimum size for the genetic alphabet?". Proc Natl Acad Sci USA 89 (7): 2614–8. Bibcode:1992PNAS...89.2614S.doi:10.1073/pnas.89.7.2614. PMC 48712.PMID 1372984.
- ^ Lindahl T (1993). "Instability and decay of the primary structure of DNA". Nature 362 (6422): 709–15.Bibcode:1993Natur.362..709L. doi:10.1038/362709a0.PMID 8469282.
- ^ Vreeland R, Rosenzweig W, Powers D (2000). "Isolation of a 250 million-year-old halotolerant bacterium from a primary salt crystal". Nature 407 (6806): 897–900.doi:10.1038/35038060. PMID 11057666.
- ^ Hebsgaard M, Phillips M, Willerslev E (2005). "Geologically ancient DNA: fact or artefact?". Trends Microbiol 13 (5): 212–20. doi:10.1016/j.tim.2005.03.010.PMID 15866038.
- ^ Nickle D, Learn G, Rain M, Mullins J, Mittler J (2002). "Curiously modern DNA for a "250 million-year-old" bacterium". J Mol Evol 54 (1): 134–7. doi:10.1007/s00239-001-0025-x. PMID 11734907.
- ^ Callahan, M.P.; Smith, K.E.; Cleaves, H.J.; Ruzica, J.; Stern, J.C.; Glavin, D.P.; House, C.H.; Dworkin, J.P. (11 August 2011). "Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases". PNAS.doi:10.1073/pnas.1106493108. Retrieved 15 August 2011.
- ^ Steigerwald, John (8 August 2011). "NASA Researchers: DNA Building Blocks Can Be Made in Space". NASA. Retrieved 10 August 2011.
- ^ ScienceDaily Staff (9 August 2011). "DNA Building Blocks Can Be Made in Space, NASA Evidence Suggests". ScienceDaily. Retrieved 9 August 2011.
- ^ Goff SP, Berg P (1976). "Construction of hybrid viruses containing SV40 and lambda phage DNA segments and their propagation in cultured monkey cells". Cell 9 (4 PT 2): 695–705. doi:10.1016/0092-8674(76)90133-1.PMID 189942.
- ^ Houdebine L (2007). "Transgenic animal models in biomedical research". Methods Mol Biol 360: 163–202.doi:10.1385/1-59745-165-7:163. ISBN 1-59745-165-7.PMID 17172731.
- ^ Daniell H, Dhingra A (2002). "Multigene engineering: dawn of an exciting new era in biotechnology". Curr Opin Biotechnol 13 (2): 136–41. doi:10.1016/S0958-1669(02)00297-5. PMC 3481857. PMID 11950565.
- ^ Job D (2002). "Plant biotechnology in agriculture".Biochimie 84 (11): 1105–10. doi:10.1016/S0300-9084(02)00013-5. PMID 12595138.
- ^ Collins A, Morton N (1994). "Likelihood ratios for DNA identification". Proc Natl Acad Sci USA 91 (13): 6007–11. Bibcode:1994PNAS...91.6007C.doi:10.1073/pnas.91.13.6007. PMC 44126.PMID 8016106.
- ^ Weir B, Triggs C, Starling L, Stowell L, Walsh K, Buckleton J (1997). "Interpreting DNA mixtures". J Forensic Sci 42 (2): 213–22. PMID 9068179.
- ^ Jeffreys A, Wilson V, Thein S (1985). "Individual-specific 'fingerprints' of human DNA". Nature 316 (6023): 76–9.Bibcode:1985Natur.316...76J. doi:10.1038/316076a0.PMID 2989708.
- ^ Colin Pitchfork — first murder conviction on DNA evidence also clears the prime suspect Forensic Science Service Accessed 23 December 2006
- ^ "DNA Identification in Mass Fatality Incidents". National Institute of Justice. September 2006.
- ^ Baldi, Pierre; Brunak, Soren (2001). Bioinformatics: The Machine Learning Approach. MIT Press. ISBN 978-0-262-02506-5. OCLC 45951728.
- ^ Gusfield, Dan. Algorithms on Strings, Trees, and Sequences: Computer Science and Computational Biology.Cambridge University Press, 15 January 1997. ISBN 978-0-521-58519-4.
- ^ Sjölander K (2004). "Phylogenomic inference of protein molecular function: advances and challenges".Bioinformatics 20 (2): 170–9.doi:10.1093/bioinformatics/bth021. PMID 14734307.
- ^ Mount DM (2004). Bioinformatics: Sequence and Genome Analysis (2 ed.). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. ISBN 0-87969-712-1.OCLC 55106399.
- ^ Rothemund PW (2006). "Folding DNA to create nanoscale shapes and patterns". Nature 440 (7082): 297–302. Bibcode:2006Natur.440..297R.doi:10.1038/nature04586. PMID 16541064.
- ^ Andersen ES, Dong M, Nielsen MM (2009). "Self-assembly of a nanoscale DNA box with a controllable lid".Nature 459 (7243): 73–6. Bibcode:2009Natur.459...73A.doi:10.1038/nature07971. PMID 19424153.
- ^ Ishitsuka Y, Ha T (2009). "DNA nanotechnology: a nanomachine goes live". Nat Nanotechnol 4 (5): 281–2.Bibcode:2009NatNa...4..281I.doi:10.1038/nnano.2009.101. PMID 19421208.
- ^ Aldaye FA, Palmer AL, Sleiman HF (2008). "Assembling materials with DNA as the guide". Science 321 (5897): 1795–9. Bibcode:2008Sci...321.1795A.doi:10.1126/science.1154533. PMID 18818351.
- ^ Wray G; Martindale, Mark Q. (2002). "Dating branches on the Tree of Life using DNA". Genome Biol 3 (1): reviews0001.1–reviews0001.7. doi:10.1046/j.1525-142X.1999.99010.x. PMC 150454.PMID 11806830.
- ^ Lost Tribes of Israel, NOVA, PBS airdate: 22 February 2000. Transcript available from PBS.org. Retrieved 4 March 2006.
- ^ Kleiman, Yaakov. "The Cohanim/DNA Connection: The fascinating story of how DNA studies confirm an ancient biblical tradition". aish.com (13 January 2000). Retrieved 4 March 2006.
- ^ Bhattacharya, Shaoni. "Killer convicted thanks to relative's DNA". newscientist.com (20 April 2004). Retrieved 22 December 06.
- ^ Goldman, Nick; Bertone, Paul; Chen, Siyuan; Dessimoz, Christophe; LeProust, Emily M.; Sipos, Botond; Birney, Ewan (23 January 2013). "Towards practical, high-capacity, low-maintenance information storage in synthesized DNA". Nature 494 (7435): 77–80.doi:10.1038/nature11875. PMC 3672958.PMID 23354052.
- ^ Naik, Gautam (24 January 2013). "Storing Digital Data in DNA". Wall Street Journal. Retrieved 24 January 2013.
- ^ Dahm R (2008). "Discovering DNA: Friedrich Miescher and the early years of nucleic acid research". Hum. Genet.122 (6): 565–81. doi:10.1007/s00439-007-0433-0.PMID 17901982.
- ^ Jones, Mary Ellen (September 1953). "Albrecht Kossel, A Biographical Sketch". Yale Journal of Biology and Medicine (National Center for Biotechnology Information)26 (1): 80–97. PMC 2599350. PMID 13103145.
- ^ Levene P, (1 December 1919). "The structure of yeast nucleic acid". J Biol Chem 40 (2): 415–24.
- ^ Astbury W, (1947). "Nucleic acid". Symp. SOC. Exp. Biol. 1 (66).
- ^ Valery N. Soyfer (2001). "The consequences of political dictatorship for Russian science". Nature Reviews Genetics2 (9): 723–729. doi:10.1038/35088598.PMID 11533721.
- ^ Lorenz MG, Wackernagel W (1994). "Bacterial gene transfer by natural genetic transformation in the environment". Microbiol. Rev. 58 (3): 563–602.PMC 372978. PMID 7968924.
- ^ Avery O, MacLeod C, McCarty M (1944). "Studies on the Chemical Nature of the Substance Inducing Transformation of Pneumococcal Types: Induction of Transformation by a Desoxyribonucleic Acid Fraction Isolated from Pneumococcus Type Iii". J Exp Med 79(2): 137–158. doi:10.1084/jem.79.2.137.PMC 2135445. PMID 19871359.
- ^ Hershey A, Chase M (1952). "Independent Functions of Viral Protein and Nucleic Acid in Growth of Bacteriophage". J Gen Physiol 36 (1): 39–56.doi:10.1085/jgp.36.1.39. PMC 2147348.PMID 12981234.
- ^ The B-DNA X-ray pattern on the right of this linked image was obtained by Rosalind Franklin and Raymond Gosling in May 1952 at high hydration levels of DNA and it has been labeled as "Photo 51"
- ^ Nature Archives Double Helix of DNA: 50 Years
- ^ "Original X-ray diffraction image". Osulibrary.oregonstate.edu. Retrieved 6 February 2011.
- ^ The Nobel Prize in Physiology or Medicine 1962Nobelprize .org Accessed 22 December 06
- ^ Brenda Maddox (23 January 2003). "The double helix and the 'wronged heroine'" (PDF). Nature 421 (6921): 407–408. doi:10.1038/nature01399. PMID 12540909.
- ^ Crick, F.H.C. On degenerate templates and the adaptor hypothesis (PDF). genome.wellcome.ac.uk (Lecture, 1955). Retrieved 22 December 2006.
- ^ Meselson M, Stahl F (1958). "The replication of DNA in Escherichia coli". Proc Natl Acad Sci USA 44 (7): 671–82. Bibcode:1958PNAS...44..671M.doi:10.1073/pnas.44.7.671. PMC 528642.PMID 16590258.
- ^ The Nobel Prize in Physiology or Medicine 1968Nobelprize.org Accessed 22 December 06
Further reading
- Berry, Andrew; Watson, James. (2003). DNA: the secret of life. New York: Alfred A. Knopf. ISBN 0-375-41546-7.
- Calladine, Chris R.; Drew, Horace R.; Luisi, Ben F. and Travers, Andrew A. (2003). Understanding DNA: the molecule & how it works. Amsterdam: Elsevier Academic Press. ISBN 0-12-155089-3.
- Dennis, Carina; Julie Clayton (2003). 50 years of DNA. Basingstoke: Palgrave Macmillan. ISBN 1-4039-1479-6.
- Judson, Horace F. 1979. The Eighth Day of Creation: Makers of the Revolution in Biology. Touchstone Books, ISBN 0-671-22540-5. 2nd edition: Cold Spring Harbor Laboratory Press, 1996 paperback: ISBN 0-87969-478-5.
- Olby, Robert C. (1994). The path to the double helix: the discovery of DNA. New York: Dover Publications. ISBN 0-486-68117-3., first published in October 1974 by MacMillan, with foreword by Francis Crick;the definitive DNA textbook,revised in 1994 with a 9 page postscript
- Micklas, David. 2003. DNA Science: A First Course. Cold Spring Harbor Press: ISBN 978-0-87969-636-8
- Ridley, Matt (2006). Francis Crick: discoverer of the genetic code. Ashland, OH: Eminent Lives, Atlas Books. ISBN 0-06-082333-X.
- Olby, Robert C. (2009). Francis Crick: A Biography. Plainview, N.Y: Cold Spring Harbor Laboratory Press. ISBN 0-87969-798-9.
- Rosenfeld, Israel. 2010. DNA: A Graphic Guide to the Molecule that Shook the World. Columbia University Press: ISBN 978-0-231-14271-7
- Schultz, Mark and Zander Cannon. 2009. The Stuff of Life: A Graphic Guide to Genetics and DNA. Hill and Wang: ISBN 0-8090-8947-5
- Stent, Gunther Siegmund; Watson, James. (1980). The double helix: a personal account of the discovery of the structure of DNA. New York: Norton. ISBN 0-393-95075-1.
- Watson, James. 2004. DNA: The Secret of Life. Random House: ISBN 978-0-09-945184-6
- Wilkins, Maurice (2003). The third man of the double helix the autobiography of Maurice Wilkins. Cambridge, Eng: University Press. ISBN 0-19-860665-6.
External links
| Library resources |
|---|
| About DNA |
| Wikiquote has a collection of quotations related to: DNA |
| Wikimedia Commons has media related to: DNA |
- DNA at the Open Directory Project
- DNA binding site prediction on protein
- DNA the Double Helix Game From the official Nobel Prize web site
- DNA under electron microscope
- Dolan DNA Learning Center
- Double Helix: 50 years of DNA, Nature
- Proteopedia DNA
- Proteopedia Forms_of_DNA
- ENCODE threads explorer ENCODE Home page. Nature (journal)
- Double Helix 1953–2003 National Centre for Biotechnology Education
- Genetic Education Modules for Teachers—DNA from the Beginning Study Guide
- PDB Molecule of the Month pdb23_1
- Rosalind Franklin's contributions to the study of DNA
- U.S. National DNA Day—watch videos and participate in real-time chat with top scientists
- Clue to chemistry of heredity found The New York Times June 1953. First American newspaper coverage of the discovery of the DNA structure
- Olby R (2003). "Quiet debut for the double helix". Nature 421 (6921): 402–5. doi:10.1038/nature01397. PMID 12540907.
- DNA from the Beginning Another DNA Learning Center site on DNA, genes, and heredity from Mendel to the human genome project.
- The Register of Francis Crick Personal Papers 1938 – 2007 at Mandeville Special Collections Library, University of California, San Diego
- Seven-page, handwritten letter that Crick sent to his 12-year-old son Michael in 1953 describing the structure of DNA. See Crick’s medal goes under the hammer, Nature, 5 April 2013.